Spectroscope Instructions

The spectroscope is used to analyze light passing through a stone. White light is a combination of all the colors of the visible spectrum: red, orange, yellow, green, blue, indigo, and violet. This is the rainbow we see when light travels through a prism. When white light travels though a stone, one or more of the wavelengths that produce color are absorbed by the gem. The colors that are NOT absorbed are the colors seen when we look at the stone.
The wavelengths that are absorbed by the stone are seen in the spectroscope as vertical black lines in the spectrum. Each variety stone has a unique absorption spectrum (like a fingerprint of the stone) When identifying a stone we look for a spectrum that is characteristic for that stone.
The wavelengths that are absorbed by the stone are seen in the spectroscope as verticle black lines in the spectrum. Each stone has a unique absorption spectrum (like a fingerprint of the stone) When identifying a stone we look for a spectrum that is characteristic for that stone.
Using a spectroscope takes practice! Try using it in different lights, with different stones. It takes some time to learn what you are looking for and what those little fuzzy lines mean. What you actually see through your spectroscope will not be as clear and pretty as what you see in books or charts.

- Use a strong incandescent flashlight (don’t forget to check the spectrum!)
- When using this method, you must restrict the light source. The only light entering the spectroscope should be coming through the stone. You can use removable putty (available at office supply stores)to block the light around the stone. Place a donut shaped piece of putty on the flashlight, the hole in the center should be about the size of the stone. If you do not have putty, you can use a piece of cardboard or other light blocking material with a hole the right size for your stone cut into it. Old business cards work well for this!
- Place the stone in the center of the donut shaped putty. Be sure that no light comes around the edges of the stone
- Holding the flashlight in one hand and your spectroscope in the other, bring the spectroscope to your eye, and then bring the flashlight holding the stone close to the spectroscope. You may need to adjust the distance between the stone and the spectroscope, reposition the stone, and try different angles.

- Use a strong incandescent flashlight (don’t forget to check the spectrum!)
- Place the stone table side down on a flat surface.
- Position the flashlight so that the light enters the stone from the side. You can use a thick book or other object and putty to hold the flashlight in place if you don’t have a stand. The light should reflect off the table facet inside the stone and bounce out through the opposite side of the stone.
- Position your spectroscope so that the light is bouncing off the table facet enters the spectroscope. You will see a bright flash where the reflection is, that’s where you want to aim the spectroscope.
Pro tips:
- Don’t give up! It takes patience and practice, and often quite a few aspirin. Repeat often: "Gemology is fun! Gemology is fun!"
- Be sure your light is full spectrum, and has no absorption lines. (Yes, this needs to be said again..and again...again...
- Make that only light from the stone is reaching the spectroscope in the transmission method.
- Practice with a stone that has a strong spectrum. Garnets are good stones to use in the beginning.
- Don’t expect the lines you see to be as strong or precise as the ones you see in books. They are going to be fuzzy, and some very faint.
- Use a light that is bright, but not too bright. You may have to try different sources until you find the one that works for you.
- Adjust the distance between the spectroscope and the stone. Different spectrums will showup better at different distances.
- Try different angles on your stone. Most spectrums will show best with the table down, but you might need to try many different angles before you find a spectrum.
- It is easier to find a spectrum in a large stone, small stones have fainter spectrums.
- The more transparent the stone is, the better.
- Stones with vivid, deep colors will have a stronger spectrum than lighter colored stones of the same variety.
- Keep your spectroscope clean and free of dust. Dust can cause horizontal lines in the spectrum.
- If you see these try blowing the dust off, don’t use your fingers!
- Don’t let your stone get to warm. If your stone has been sitting on the light source too long and gotten warm, it will be harder to see the spectrum. Take off the light source and let it cool off for a while, then try again.
- Practice, practice, and then practice some more.
Not all stones can be identified through their spectrum. You should always use at least 3 tests when identifying a stone. Some of the areas the spectroscope is helpful with:
* Separating natural and synthetic ruby/emerald from glass or other imitation stones. It will NOT help you separate natural from synthetic ruby and emerald
*Detecting dye in jadeite or chalcedony
* Separating synthetic blue spinel from aquamarine, zircon, and blue sapphire
* Identifying demantoid, almandite, pyrope-almandite, pyrope garnet, synthetic blue spinel, natural blue spinel, red spinel, zircon, peridot, and chrysoberyl (other than alexandrite)
* Some treatments in diamond
|
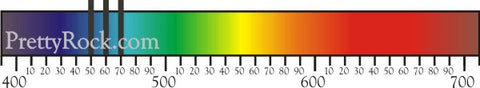
Absorption Spectrums for Common Gemstones Below is a quick reference of simulated absorption spectrums for common gemstones. There are many different signature spectrums within each type of gem depending on locality, depth/saturation/hue of color, and of course the specific chemical impurities that happen to affect the gemstone's color. This quick reference gives you a generic idea of what light frequencies will be absorbed for each gemstone. We strongly recommend purchasing one of many great textbooks in order to comprehensively and accurately identify your gemstones. Another great online source for spectrum images is: Spectra Database The wavelength nanometer scale (the numbers below the spectrum) is below the spectrum. NOTE: Most spectroscopes do NOT include a wavelength scale. That is a feature reserved for expensive professional equipment. The absorption lines have been stretched above the spectrum for quick and easy reading. |
|
Blue Sapphire |
Ruby |
Emerald |
Aquamarine |
Blue Spinel |
Red Spinel |
Peridot |
Rubellite Tourmaline |
Blue Green Tourmaline
|